Kinghawk Pharmaceutical's COVID-19 nucleic acid test kit approved for listing
The COVID-19 nucleic acid test kit developed by Zhongguancun enterprise Beijing Kinghawk Pharmaceutical Corporation [Photo provided to chinadaily.com.cn]
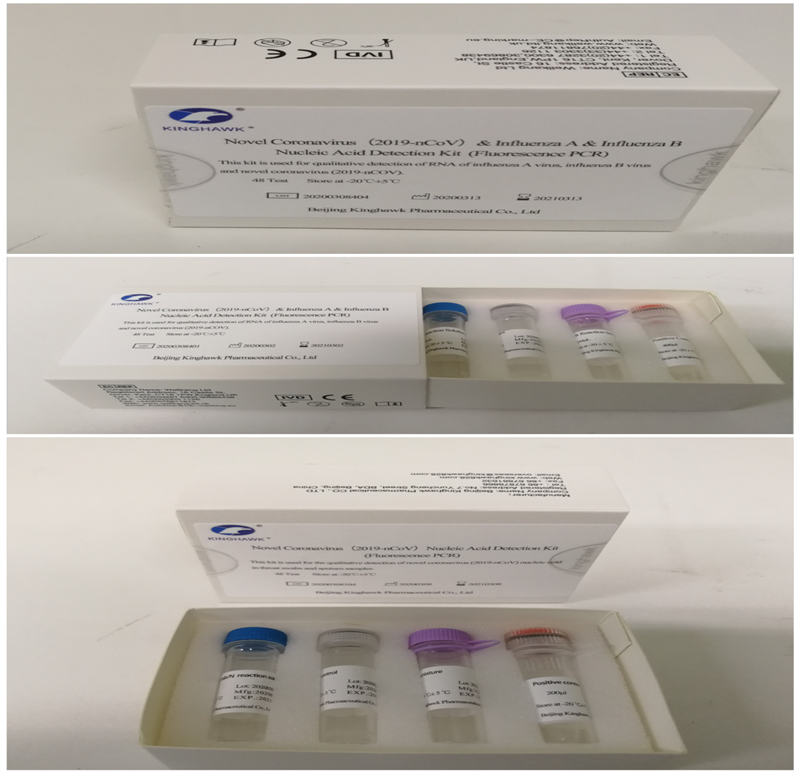
The COVID-19 nucleic acid test kit (fluorescent PCR method) developed by Zhongguancun enterprise Beijing Kinghawk Pharmaceutical Corporation (“Kinghawk Pharmaceutical”) has passed review by the National Medical Products Administration and CE certification.
As of now, the test kits developed by six Zhongguancun enterprises have been approved by the National Medical Products Administration.
The Kinghawk Pharmaceutical designed a specific primer and Taqman probe for the target genes of COVID-19, the ORF1ab and N genes. It has a high level of detection of COVID-19 through the fluorescent PCR detector. The kit uses the mRNA of ribonuclease P widely present in normal human epithelial cells as a control for those cells.
Prior to this, clinical trials of the kit had been completed in three clinical units, using throat swabs and sputum as samples. Using the disease diagnosis / exclusion criteria specified in the "Diagnosis and Treatment Protocol for COVID-19", this kit has a sensitivity of 91.34 percent and a specificity of 100 percent; the total coincidence rate is 92.65 percent compared with similar kits that have been approved for listing.
"This kit can be used for in vitro qualitative detection of the ORF1ab and N genes of COVID-19 in throat swab and sputum samples, as well as auxiliary diagnosis and epidemiological monitoring of COVID-19 infection. The detection results can be obtained within 90 minutes," said Zhang Zhi, CEO of Kinghawk Pharmaceutical.
As early as the 2009 H1N1 influenza outbreak, Kinghawk Pharmaceutical developed a H1N1 influenza virus (2009) RNA test kit (fluorescence PCR method) in only 53 days. It has produced over 500,000 such kits, which are applied in 224 monitoring spots in China.
Kinghawk Pharmaceutical has developed a number of genetically engineered antigens and monoclonal antibodies, and released more than 80 products including an enzyme-linked immunosorbent assay kit, a rapid immune diagnostic reagent, a molecular diagnostic reagent, a blood grouping reagent, and a biochemical reagent.
