13th Five-Year Plan energizes ZGC'S bio-health industry
During the 13th Five-Year Plan period, the bio-health industry in Zhongguancun Science Park proliferated.
As of 2019, Zhongguancun is home to more than 2,300 companies in the biological health field, with an industrial scale of 258.4 billion yuan ($ 39.56 billion), a year-on-year increase of 15.6 percent.
These enterprises' research and development expenditures reached 18.1 billion yuan, a four-year compound annual growth rate of more than 10 percent. Meanwhile, patent applications in such fields amount to 3,584, a four-year compound annual growth rate of 8.9 percent.
Independent innovation enters the harvest season
A 91-year-old man with fractured bones had successful orthopedic surgery this September. The assisting doctor was an orthopedic surgical robot independently developed by Tianzhihang called "Tian Ji."
Orthopedic surgery requires exceptionally high precision. The assistance of surgical robots can improve the accuracy, shorten the operation time and realize smaller wounds, less bleeding and faster recovery.
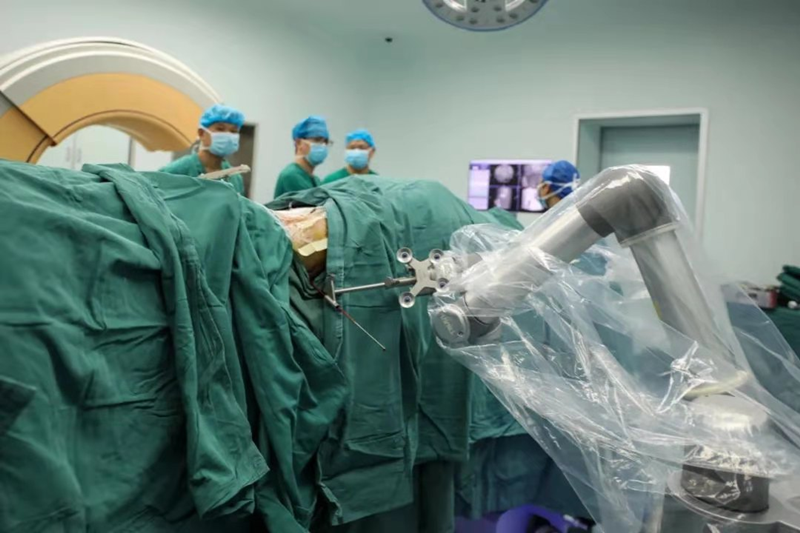
"Tian Ji" orthopedic surgical robot performs surgery for patients. [Photo provided to chinadaily.com.cn]
As a pioneer of domestic surgical robots, TINAVI has developed the only orthopedic surgical robot in the world capable of performing traumatic orthopedics and spine surgery.
The robot has completed more than 8,000 surgeries in more than 80 medical institutions across the country, benefiting many patients.
In July 2020, TINAVI was listed on the Science and Technology Innovation Board, becoming the first domestically listed surgical robot company.
In Zhongguancun, a group of innovative medical device R&D companies – including Sinovation, Remebot and Shurui – is also making steady progress on the journey of domestic substitution of high-end medical devices.
In addition to intelligent surgical robots, the development of innovative drugs is another eye-catching one.
For a long time, the anti-cancer drugs marketed in China were primarily imports, but a new anti-cancer drug born in Zhongguancun successfully addresses this situation.
After more than 2,600 days and nights of research and development, BeiGene's self-developed BTK inhibitor zanubrutinib was approved by the U.S. Food and Drug Administration to be listed on the US market as China's first self-developed anti-cancer drug in November 2019.
As a targeted medicine, Zebutinib can treat a variety of B-cell malignancies, including mantle cell lymphoma, and 84 percent of the treated patients realized overall remission.
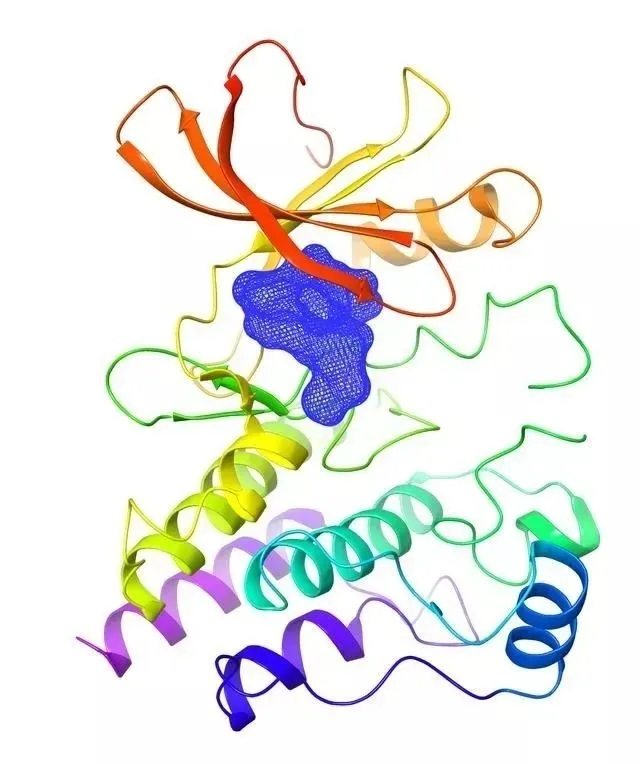
A schematic diagram of the crystal structure of Zebutinib and BTK protein complex. [Photo provided to chinadaily.com.cn]
Zhongguancun enterprises have mastered technological leadership in many fields, such as targeted drugs, new combined vaccines, antibody preparation and interventional implant material technology.
Huahui Health, which focuses on drugs to treat liver diseases, has developed a new anti-hepatitis B monoclonal antibody HH-003 with global intellectual property rights and is regarded as "the gospel of hepatitis B patients."
Bosis Healing independently develops non-cross-linked SIS biological materials, becoming the second company in the world to realize the industrialization and full-line product layout of such materials.
Lepu Medical launched a fully degradable stent in 2019, which is China's only absorbable stent product in use.
By November 2020, Zhongguancun had been approved to produce seven Class I new drugs, and nearly 20 companies' Class I new drugs have entered Phase II clinical trials. A total of 25 innovative medical devices have been approved, accounting for about one-third of those in China.
Hard-core industrial cluster
In 2010, Xie Jiangbing, the founder of Eyebright, settled in Zhongguancun and devoted himself to the R&D of intraocular lenses, a biological material required for eye surgery.
After four years of exploration, Eyebright has created a domestic soft folding intraocular lens with independent intellectual property rights. The product was recognized internationally as a third-generation aspheric technology and quickly entered high-end medical devices with its high quality and low price, bringing light and hope to countless cataract patients.

Eyebright third-generation proming aspheric toric intraocular lens. [Photo provided to chinadaily.com.cn]
There are south and north bio-health industry clusters in Zhongguancun: the southern high-end industrial base with Yizhuang and Daxing biomedical bases as the core, and the northern R&D and innovation center with Zhongguancun Life Science Park as the center.
In the national biomedical park appraisal by the Biological Center of the Ministry of Science and Technology, the Zhongguancun Science Park has ranked first in the country for many years in terms of comprehensive competitiveness.
From laboratory to market, it takes considerable effort for a drug to finally reach patients. Although many companies have R&D capabilities, they lack development and production capabilities.
JOINN Biologics is a CDMO company specializing in the development of biological drugs. It can provide one-stop services for new drug R&D companies from cell line construction, feasibility studies, quality testing and pilot trials to commercial production.
"Now, R&D companies only need to provide a molecular sequence, and we can complete the entire process before the launch of a new drug," said Chen Baolu, global chief operating officer of JOINN Biologics.
Shuimu Medical has built the first domestic market-oriented professional service platform for the entire chain of medical device testing services, which provides medical device R&D companies with non-functional testing services. It offers professional services for domestic medical device products under research.
With 13 technology transfer service platforms, 41 open laboratories, 24 bio-health field incubators and six bio-medicine characteristic parks, the Zhongguancun bio-health industry provides companies settled in the park with comprehensive services, including incubation, transformation, R&D, pilot and OEM.
Innovation empowers smart medical system
In the field of smart medical care, Zhongguancun has also made effective explorations in telemedicine, AI diagnosis, medical voice input, disease risk prediction and contactless services.
InferVision is one of the earliest companies in China to apply artificial intelligence to the field of medical imaging.
Having obtained qualifications for market access in major medical markets, it is also the only AI medical company in the world with the four major certifications of China NMPA, EU CE, Japan PMDA and US FDA.
During the outbreak of COVID-19, InferVision took the lead in launching an AI product for the disease in China – the pneumonia intelligent auxiliary screening and epidemic monitoring system, which was applied on the frontlines of the battle in Wuhan.
Since then, this A.I. product has landed in more than 50 domestic hospitals to fight the epidemic and entered hospitals in more than 20 countries, including nations in North America, Europe and Asia.
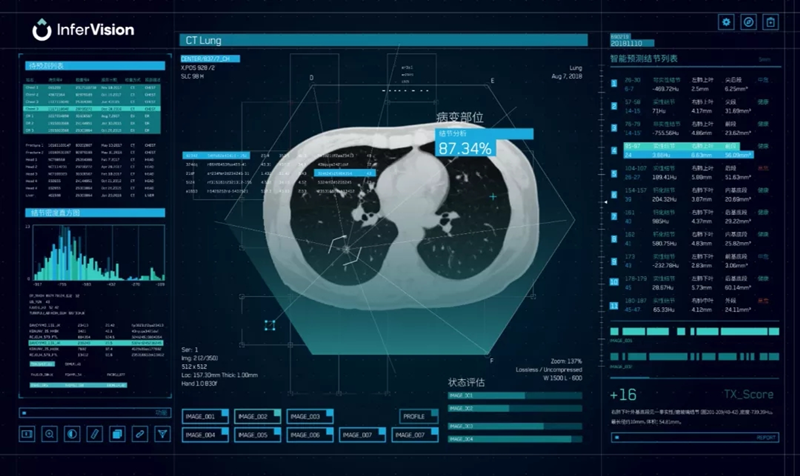
InferVision's Intelligent auxiliary screening and epidemic monitoring system interface for COVID-19 [Photo provided to chinadaliy.com.cn]
Unisound's "Intelligent Voice Electronic Medical Record System" presents another example of "cross-border integration."
The system was brought to the frontline by the first batch of medical staff in Shanghai to support Wuhan this February, bringing convenience to medical staff wearing thick masks, rubber gloves and heavy protective clothing.
Subsequently, Unisound successively launched a range of AI products such as "epidemic prevention robots," "contactless smart elevators" and "intelligent online education solutions," which were applied in Beijing, Shanghai, Hubei, Shandong, Fujian, Guangxi, Sichuan and other places.
As of November 2020, Zhongguancun had obtained four Class III medical device registration certificates in AI medicine, ranking first in the country.
During the 14th Five-Year Plan period, the bio-health companies in the Zhongguancun Science Park will make further breakthroughs in technology products, adding more prosperity to the park.
